Intermolecular Forces
- Polar Bonds vs. Polar Molecules
- A molecule is polar if the polar bonds do not "cancel out"
when you add them up (vector addition).
- CF4 is
tetrahedral in electron geometry and tetrahedral in molecular
shape.
 All four bonds are C-F bonds and are very polar YET the bond dipole arrows all cancel out.
The overall molecule is nonpolar despite the polar bonds.
All four bonds are C-F bonds and are very polar YET the bond dipole arrows all cancel out.
The overall molecule is nonpolar despite the polar bonds. - NH3 is
tetrahedral in electron geometry and trigonal pyramid in molecular shape.
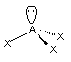 All three N-H bonds are polar
AND the bond dipole arrows do NOT cancel out. The overall molecule
is polar and has a dipole moment (a north and south pole)
All three N-H bonds are polar
AND the bond dipole arrows do NOT cancel out. The overall molecule
is polar and has a dipole moment (a north and south pole) - Check for yourself that CO is polar and CO2 is
nonpolar despite having polar bonds.
- Basically polar
molecules are those who have polar bonds arranged
asymmetrically.
- Great
animation and example problems - make sure you click next (IMPORTANT)
- Intermolecular Forces (IMF)
- Atoms are held together
in a molecule by covalent bonds. But what holds molecules with each
other? IMF! IMF are all electrostatic attractions (+ attracted
to -) Check out this tutorial - scroll down to
Intermolecular Forces Tutorial
- The IMF of gases are
<<< liquids < solids. Solids are held together very
well and it takes lots of energy to pull them apart. Liquids are
held closely together, just not in a rigid pattern, but the forces are
still significant. Gases are already apart - hardly any forces exist
between the molecules and atoms of a gas.
- Remember than ionic and
covalent bonds are far stronger than IMF.
- The IMF from strongest
to weakest are: (ALL IMPORTANT)
- Ion-dipole force: This occurs
when ions and polar molecules are together.
Cations (+) attract the d- end of the polar molecules and anions (-) attract the d + end of the polar molecules. This is why some ionic solids dissolve in water. The sum of the ion-dipole forces of the ions surrounded by water is greater than the original ionic bond. So the ionic bond breaks and the water molecules surround the ion. Why does salted water boil at a higher temperature than plain water??? Think about it.
- H bridging force. H bridges are
not really bonds but a force. This is a special kind of dipole-dipole
attraction.
When an H is covalently bonded to an N, O or F it can be attracted to a lone pair on another molecule. This is because the H-F, H-N, and H-O bonds are very polar with the H being d + and the N, O, F being d-. So the H is attracted to the negative lone pair on another molecule. Thank goodness for the H bond force in water. Because of the H bond force water is held together stronger than expected and it has a higher mp and bp than expected. In general the more mass the higher the mp and bp.
- bp of H2O is
actually higher than the more massive H2S which is abnormal
- bp of NH3 is
actually higher than the more massive PH3 which is abnormal
- bp of CH4 is
lower than SiH4 which is normal (methane can’t H bond – you
should know why!)
- bp of HF is actually
higher than the more massive HCL which is abnormal
- Water, ammonia and HF
have abnormally high mp and bp despite small mass because they have H bond
forces!!!
- Dipole-dipole force. When polar
molecules are attracted to each other.
 Consider H-Cl. The Cl is more EN than H so it
pulls the 2 electrons in the polar covalent bond closer and is d- thus the H is d +. When another
H-Cl gets close they arrange themselves so the Cl of the first molecule
is close to the H of the second. As polarity increases, the IMF increase
and the mp and bp
increase. This is because the stronger the IMF the harder it is to
pull these molecules apart so a higher temperature is needed for melting
and boiling.
Consider H-Cl. The Cl is more EN than H so it
pulls the 2 electrons in the polar covalent bond closer and is d- thus the H is d +. When another
H-Cl gets close they arrange themselves so the Cl of the first molecule
is close to the H of the second. As polarity increases, the IMF increase
and the mp and bp
increase. This is because the stronger the IMF the harder it is to
pull these molecules apart so a higher temperature is needed for melting
and boiling. - London force. This force is
in nonpolar molecules and neutral atoms. It is weak and
temporary. Consider Xe atoms which are
big and fat. Let’s say the electrons for a moment are mostly on
one side – then that side would be temporarily d- and the side with less electrons would be d+. Now an atom
next to this one would have it’s
electrons attracted to the d+ side so would also
become temporarily polar – we say it has an induced dipole. Then
it could induce a dipole in the next atom and so forth. All atoms
and molecules have London forces, but the stronger forces overshadow
them. These induced dipoles are short lived. London forces
are best in big fat atoms and molecules with lot of electrons. Polarizability
= how easy it is to induce a dipole. Polarizability thus increases
the more massive an atom. Consider the table below. The
halogens all have only London forces since they are nonpolar. Thus
as the polarizability increases (mass increases) the London forces
increase and the bp increases. Again this
is because the more London forces, the harder it is to pull them
apart. Fluorine and chlorine with the least London forces are
gases, then iodine with the most force is
actually a solid. One last thing to mention – long chain molecules can
wrap around each other thus making them harder to separate than rounder
branched chains.
|
|
F2 |
Cl2 |
Br2 |
I2 |
|
State |
gas |
gas |
liquid |
solid |
|
bp (oC) |
-188 |
-35 |
59 |
185 |
- Overall London forces
< dipole-dipole < H bond force < ion-dipole
- Put in increasing bp order: HBr, Ar, CH3OH, He Answer is
He < Ar < HBr
< CH3OH
- Liquids
- Viscosity = thickness
of a liquid (oil is thick). As IMF increases viscosity increases
because the molecules will hold together stronger making the liquid
thicker.
- Surface tension = how
the molecules hold each other at the top of a liquid. As IMF
increases the surface tension also increases because the molecules will
hold together stronger making the liquid surface tension higher.
-
- Phase Changes
- There are six phase
changes or changes in states of matter

- s g l = melting
- l g g = boiling,
evaporation, vaporization
- s g g = sublimation
- g g s =
deposition
- g g l =
condensation
- l g s = freezing
- As we go from solid to
liquid to gas, we are breaking the IMF that hold
the molecules together. Because this is just a physical change, the
molecule itself is not changing - ie the BONDS
holding the molecule together are staying intact.
- As we add heat to melt
or boil something the enthalpy is increasing, DH is + and feels cold
as the heat is going into the substance. As we remove heat to
freeze or condense something the enthalpy is decreasing, DH is - and the heat is
exiting. A freezer sucks the heat out of something to cool it down. (IMPORTANT) Don't worry about DG or DS at this time.
- Heating Curves (IMPORTANT) are graphs that show
heat on the x axis and Temperature on the y axis. Watch
this movie, go to activities and click on Changes of State Movie.
Refer to the curve at the right. As heat is added a solid will warm
up (A) until it melts, then the T stays constant while all the solid
turns to liquid (B), finally the liquid heats up (C) until it boils, then
the T stays constant while all the liquid turns to gas (D), then finally
the gas heats up (E).
- Why does the T stay
constant during a phase change? Well all the energy is going into
breaking or forming the IMF instead of kinetic energy which affects
Temperature. For example if melting, the energy is going into
breaking apart the solid so a liquid can form.
- At (B) solid is in
equilibrium with liquid
- At (D) liquid is in
equilibrium with gas
- DHfus is the heat of fusion
= energy to convert from solid to liquid
- DHvap is the heat of
vaporization = energy to convert from liquid to gas
- DHfus is always less than DHvap because it takes more
energy to completely break the IMF to turn a liquid into a gas.
- Evaporation, Vapor Pressure (VP) and Boiling Point (bp)
- Boiling = when liquid
turns to gas due to heat being applied. Boiling is a phase change
that occurs at the boiling point, which is a temperature.
- Evaporation is when
liquid turns to gas slowly at a temperature less than the bp. When a high kinetic energy molecule at the
surface of a liquid gets bumped by other molecules, it can pop out of the
liquid and join the atmosphere then float away. Evaporation thus
proceeds slowly. When the higher KE molecules leave, the lower KE
molecules are left behind so it feels cool. This is why when you
get out of the pool or shower you feel cold at first - all the
evaporation from your skin.
- Vapor Pressure = the
pressure in a closed container due to the liquid evaporating and setting
up equilibrium with its vapor. Watch
this movie. Consider an empty container - put a liquid inside -
close the lid. What is above the liquid? Well air and pressure due
to the air Pair. Now let it sit. After a while some of the
liquid has evaporated and joined the air so the pressure is greater than
it starter. The additional pressure is the VP. Now the total
pressure in the container is Pair + VP. Equilibrium is
reached: everytime a liquid molecule
joins the vapor somewhere else a vapor molecule joins back with the
liquid. (l) D (g) equilibrium. (IMPORTANT)
- Compare
all three with pictures (IMPORTANT)
- As IMF increase, the
evaporation rate will decrease because it will be harder to pull them
apart from the liquid. As IMF increase the VP will decrease for the same
reason, less will go into the vapor phase if they are held together with
stronger force. Finally as IMF increase the boiling point will
increase because it will take higher energy at higher T to pull them
apart.
- As T increases,
evaporation rate will increase, VP will increase
because with more KE the liquid molecules are more likely to join the vapor.
However bp stays constant.
- Normal bp is at 1.0 atm
(atmosphere) of pressure which is like sea level pressure - normal Earth
surface pressure. On super high mountains the pressure is less than
1.0 atm, and the bp
will decrease. With less atmosphere pushing down on liquids, they
are more likely to pop out into the air. It totally sucks to cook up high
in the mountains because water will not boil as hot as normal.
 Instant coffee is disgusting.
Instant coffee is disgusting. 
- Solids
- There are a total of 5
types of solids: (IMPORTANT)
- Ionic - held together
by ionic bonds in all directions (sometimes called ion-ion forces), high
mp and bp,
brittle. Example: NaCl and other salts.
- Molecular - held
together by IMF, low mp and bp, insulators. Examples: ice, dry ice,
solid iodine
- Covalent Network -
held together by covalent bonds in all directions, high mp and bp, super hard,
only molecule you can see with your bare eyes, they are one giant
molecule. Example: diamond, ruby
- Metallic - held
together by metallic bonds, medium to high mp
and bp, conductors, malleable, ductile.
Example: gold, copper, sodium, zinc
- Amorphous, polymers -
held together by covalent bonds, high mp but usually decompose
instead. Example: plastics, rubber
- Phase Diagrams (IMPORTANT)
- These are plots of P vs
T.
At high pressures and cold T solids exist. At low pressures and hot T gases exist. Liquids are in between. Now look at these points. Check out this tutorial - scroll down to Phase Diagrams Tutorial
- This is the triple
point. All three phases of matter can exist here in equilibrium. This
combination of P and T is the only way all three states can exist at
once and is unique for each chemical.
- This point is on the
boundary between solid and liquid and is at 1 atm
so must be the normal melting point.
- This point is on the
boundary between gas and liquid and is at 1 atm
so must be the normal boiling point.
- This is the critical
point where no matter how hard you smush
(increase pressure) you can't make a liquid at this temperature.
Everything past point 4 is a super critical fluid.
- Note the segments
between the phases are where phase changes occur. There is the
melting/freezing boundary of solid and liquid, there is the
boiling/condensing boundary of liquid and gas, and there is the
subliming/depositing boundary of solid and gas.
- The phase diagram for
CO2 looks like the one above except the 1 atm
line is below the triple point. What does that mean? Well
than at normal pressure here on Earth, the solid goes straight to the gas
(ie it sublimes) and bypasses the liquid states
completely. You would have to increase pressure to above the triple
point in order to see liquid CO2.
- Water's phase diagram
is weird. You should know exactly what is weird and what that means
for water. Go to activities and click on Phase
Diagram of Water Movie.
Study Hard.