Atomic Theory

- Law
of Conservation of Mass
- For all changes in
matter, the mass before and after is the same. Matter cannot be
created nor destroyed. Priestley and Lavoisier (1774). This
was actually hard to figure out. After all when you burn something
it seems to disappear leaving only bits of ash which weigh less than what
you initially had. Well the other products of fire are gases that
drifted away - so you didn't get their mass. But the mass of
burning something is the same before and after.

- Chemical changes can be
called chemical reactions. What you start with are
called reactants. What you end with are
called products. For chemical reactions the reactant mass = product
mass.
- Law of definite
proportions - pure samples always have the same
elemental proportions by mass. Law
of multiple proportions (Click on activities then multiple
proportions movie) - pure samples always have the same elemental
proportions by atoms. For example, the mass of H in water compared to the
mass of O in water is always the same - 1 to 8. And water is always two H
atoms per one O atom. Compare to hydrogen peroxide H2O2.
The mass of H in hydrogen peroxide compared to the mass of O in water is
always the same - 1 to 16. And hydrogen peroxide is always two H atoms
per two O atoms. Just because water and hydrogen peroxide are made
of the same two elements does not mean they are the same or even
similar. Water and hydrogen peroxide are quite different.
- Dalton's
Atomic Theory (1808)

- John Dalton's theory is
still largely true today (IMPORTANT)
- Elements are made of
tiny atoms.
- All atoms of the same
element have the same mass.
- Elements combine in
whole number ratios to make molecules and compounds.
- The number and type of
atoms before a reaction will be the same after the reaction - what changes is how the atoms are combined. (must follow
the Law of Conservation of Mass after all )
- Only one of the 4
statements above is now considered false. Which one?
- It is the second
one. All atoms of the same element don't have the same mass because
of isotopes.
- Electrons
- Thomson discovered
electrons in the 1890's in cathode
ray tube experiments. (Click on cathode ray tube) If you
connect an evacuated glass tube with two pieces of metal at each end to a
battery eventually electrons will pass from the negative metal to the
positive metal. This electron beam must be negatively charged since
it goes to the positive side. This is how a TV works. See figure 2.3 in
your text. We also know the electron beam is charged because a magnet can
deflect the beam from traveling in a straight line.
- Millikan
(Click on Milikan oil drop) later found that
the charge on an electron must be -1.6 x 10-19 Coulombs and
the mass of an electron is 9.11 x 10-28 grams.
- Protons
and Neutrons
- Matter is neutral
overall so if electrons in atoms are negative there must be a positive
part also.
- Rutherford
(Click on activities then Rutherford movie) knew that Ra, Po, and Rn all
emit alpha particles that have a +2 charge. So he set up an
experiment where alpha particles would pass through gold foil. He
thought that all would pass but to his surprise some alpha particles were
deflected. He decided that an atom was mostly empty space except
for a tiny nucleus that held a positive charge and most of the mass. See
figure 2.5 in your book.
- If the nucleus of an
atom were a marble, the atom would be the size of the Cardinal's stadium.
The protons and neutrons live inside the nucleus. (IMPORTANT)
- protons have +1 charge
and mass = 1.67 x 10-24 g which is ~ 1 amu
(atomic mass unit)
- neutrons have 0 charge
and mass = 1.67 x 10-24 g which is ~ 1 amu
- electrons have -1
charge and mass = 9.11 x 10-28 g which is ~ (1 / 2000) amu (negligible mass)
- Electrons go around the
proton and neutron containing nucleus. Mass is due to the protons
and neutrons. Size is due to the electrons. Charge is due to
the protons and electrons. Atoms are neutral so the number of protons
must equal the electrons. (IMPORTANT)
- Example: Tommy
dropped a thermometer in lab. The mercury made a drop with a
diameter of 2.5 cm. A mercury atom is about 2.98 x 10-10
m in diameter. How many mercury atoms across is this drop? Try
first before looking at the answer!!!
- Answer: 2.5 cm ( 1 m / 100 cm) = 0.025m across the drop. How
many atoms will fit? 0.025 m (1 atom / 2.98 x 10-10 m) =
8.4 x 107 Hg atoms.
- Atomic
Number

- What makes atoms
different from each other? Well it is their number of protons which
we call the atomic number. The # protons
determines the identity of an atom, not electrons, not neutrons,
not mass - just number of protons!!! (IMPORTANT)
- Atomic mass
- well what has mass in an atom??? Mainly protons and
neutrons. So atomic mass = # protons + neutrons.
- Isotopes =
same element, so same # protons, but different mass, so different #
neutrons. (IMPORTANT)
- 1H and 2H
and 3H are isotopes of hydrogen. What is the
same? one proton What is
different? # neutrons
- 12C and 13C
and 14C are isotopes of carbon. What is the same?
6 protons What is different? #
neutrons
- What
element has 7 protons? nitrogen
- What
element has 10 neutrons? we don't know, identity of an atom
depends on # protons only
- What
element has 8 electrons? we don't know, identity of an atom
depends on # protons only
- If I add one neutron to a carbon atoms what element to I have?
carbon (did I get you?)
- Practice problems
- How many protons,
neutrons and electrons are in: 28Si, 14C
and 11B?
- Fill in this table
for ATOMS:
|
Symbol |
127Xe |
|
12C |
|
41Ca |
1H |
|
|
#
protons |
|
38 |
|
|
|
|
1 |
|
#
neutrons |
|
49 |
|
8 |
|
|
1 |
|
#
electrons |
|
|
|
6 |
|
|
|
- answers to a above: 28Si
- 14p, 14n, 14e, 14C - 6p, 8n, 6e and 11B - 5p,
6n, 5e
- answers for the
table:
|
Symbol |
127I |
87Sr |
12C |
14C |
41Ca |
1H |
2H |
|
#
protons |
54 |
38 |
6 |
6 |
20 |
1 |
1 |
|
#
neutrons |
73 |
49 |
6 |
8 |
21 |
0 |
1 |
|
#
electrons |
54 |
38 |
6 |
6 |
20 |
1 |
1 |
- Atomic
Mass
(ignore the mole for now)
- The atomic mass and
atomic number are given on the periodic table. The units for mass
are amu. So why aren't the masses whole
numbers? You can't have "part" of a proton or
neutron? Well the masses are averages for all the isotopes.
- Carbon is 12.011 amu. That is mostly carbon-12 plus a little bit
of carbon-13. Thus the average is a little higher than 12.
- To calculate the atomic
mass sum the mass of each isotope times the percentage of that
isotope.
- Compounds
and Mixtures
- Definitions (IMPORTANT)
- Element - sample of
one type of atom
- Molecule - when 2 or
more atoms are bonded together
- Compound - when 2 or
more different atoms are bonded together
- Pure - each species or
unit in the sample is the same (every molecule is the same for example)
- Mixture - 2 or more
different atoms or molecules, opposite of pure
- Homogeneous -
uniformly mixed, can't see the separate species (salt water, coffee)
- Heterogeneous - not
uniformly mixed, can see the separate components (granite, dirt)
- Questions to try
- What is air? A
mixture
- What is tap
water? a mixture
- What is DI
water? pure, compound, molecules
- What is O2, oxygen gas? pure,
element, molecule
- Look -
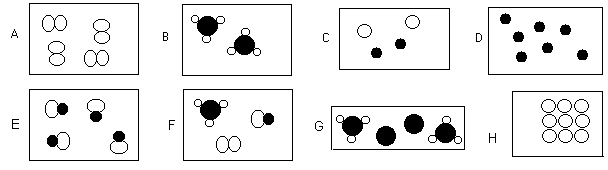
- Are the pictures above
elements, compounds, pure, mixtures, or molecules.
More than one may apply.
(IMPORTANT)
- molecules, pure,
element
- pure, compound,
molecules
- mixture
- pure, element
- pure, compound,
molecules
- mixture
- mixture
- pure, element
- Ions and Covalent Bonds
- Chemistry is all about making and breaking
bonds in chemical reactions. Bonding is all about electrons. (IMPORTANT)
- For main
group elements, bonds are either covalent or ionic
- Covalent (IMPORTANT)
- electrons
are shared between nonmetals and other nonmetals
- the
nonmetals may be the same element or different elements
- molecules
are 2 or more atoms held together by covalent bonds (H2O,
CO, CO2, H2O2, O2, N2)
- Diatomic
elements are molecules - these elements occur naturally paired up (H2,
N2, O2, F2, Cl2, Br2,
and I2) They will not be found in
nature as individual atoms. (IMPORTANT)
- Ionic (IMPORTANT)
- electrons
are transferred from metal atoms to nonmetal atoms
- the
metals lose negatively charged electrons so they become positively
charged cations
- the
nonmetals gain negatively charged electrons so they become negatively
charged anions
- Opposites
attract, so the cations and anions hang out
 and become ionic solids
and become ionic solids - There is
no real "molecule" here - the ions just keep packing into a
nice pattern for the solid
- Polyatomic
ions - several atoms held together by covalent bonds but they have an
overall charge so as a group make an ion and can form ionic bonds with
other atoms. More on them later.
- Ionic or
Covalent? H2O, CaCl2, KBr,
N2, Cu, HCl, H2O2
Answer: H2O, N2, HCl,
H2O2 are
covalent. The rest are ionic except Cu which is neither since it
is not bonded!!!