General Chemistry Help
![]()
![]()
![]()
![]()
![]()
![]()
|
We have Free
Tutoring – Ask your instructor when and where |
||
Math Help / Problem Solving – downloadable lessons on the left margin
Electron
dot structures tutorial
Electron
dot structures practice probs
Stoichiometry (practice problems like grams to grams)
Oxidation states, ionic formulas and polyatomic ions
Writing and balancing chemical reactions
Chem Tutor - help for many basic topics including math help
Chem Web - graphics intensive - great reviews, has interactive quizzes, fun
Interactive Periodic Table - info on most every element
|
Oxidation states, ionic formulas and polyatomic ions Molarity and dilution (practice problems) Quantum numbers, Shells, Orbitals Chem Tutor - help for many basic topics including math help Chem Web - graphics intensive - great reviews, has interactive quizzes, fun Gen Chem Topic Review - Purdue has great info for 151 students Practice Problems by Topic very helpful
|
Writing and balancing chemical reactions Stoichiometry (practice problems) Oxidation and Reduction basic info Interactive Periodic Table - info on most every element Math Help / Problem Solving – downloadable lessons on the left margin |
|
Chem Tutor - help for many basic topics including math help Chem Web - graphics intensive - great reviews, has interactive quizzes, fun Interactive Periodic Table - info on most every element Gen Chem Topic Review - Purdue has great info for 151 and 152 students Dr. Diebolt's help page - CHM 152 info for her sections Math Help / Problem Solving – downloadable lessons on the left margin |
Acids, Bases
and their equilibria Buffer and Titration Activities
|
Table of Acid, Base and Salt
Information
|
Chemical |
Example reaction |
Equili brium? |
In the beaker (picture) |
|
Strong acid |
HCl(aq) +
H2O(l) g
Cl- (aq) + H3O+(aq) |
no |
Cl-, H+ |
|
Weak acid |
HF (aq)
+ H2O(l) D F- (aq) +
H3O+ (aq) |
yes |
HF, F-, H+ |
|
Strong base |
NaOH(s)
g Na+ (aq) + OH- (aq) (does not react with water, dissolves
in it) |
no |
Na+, OH- (products only) |
|
Weak base |
NH3(aq)
+ H2O(l) D OH- (aq) +
NH4+ (aq) |
yes |
NH3, OH-, NH4+
(both sides) |
|
Acidic salt |
NH4Cl g
NH4+ (aq) + Cl- (aq) (does not react with water, dissolves
in it) then the non neutral ion (conjugate acid) reacts
further with water NH4+ (aq) + H2O(l) D NH3(aq) + H3O+
(aq) |
yes |
NH3, H+ , NH4+
Cl- (both sides) |
|
Basic salt |
KF g
K+ (aq) +
F- (aq) (does not react with water, dissolves
in it) then the non neutral ion (conjugate base )
reacts further with water F- (aq)
+ H2O(l) D HF (aq) + OH- (aq) |
yes |
HF, F-, OH-, K+ (both
sides) |
|
Neutral salt |
NaCl g
Na+ (aq) +
Cl- (aq) (does not react with water, dissolves
in it) both ions are neutral so no
further reaction |
no |
Na+, Cl- (products only) |
Significant digits are not something we make up to terrorize you all semester long. They represent the accuracy of a measurement. For example, a cheap bathroom scale bought at the dollar store reads your weight as 152 pounds, not 152.45809 pounds. It is not that accurate. Significant digits are very important in all measurements. Now remember that conversion factors such as (1 L / 1000 mL) DO NOT affect the significant digits because they are exact numbers.
- Rules - note that # means a non-zero digit (123456789)
- Zeros in front of any #
never count
- Zeros after any # do
not count unless they are also after a decimal place
- Zeros in between any
digits that count, count also
- Examples
- 2040 - 3 sig dig
- 2040.0 - 5 sig dig
- 00204.0 - 4 sig dig
- 0.00204 - 3 sig dig
- 0.020400 - 5 sig dig
- 2.0400 - 5 sig dig
- 100,000 - 1 sig dig
- 100,000.0 7 sig dig
- 0.0001 - 1 sig dig
- 0.000100 - 3 sig dig
- When multiplying or dividing, the answer must have the
same number of sig dig as the least sig dig in the problem. Your
answer can not be more accurate than any measurement in the problem.
- When adding or subtracting the answer must have the
same number of decimal places as the least number of decimal places in the
problem. Your answer can not be more accurate than any measurement in the
problem.
- Example: Pretend we weigh something on a cheap
scale and it is 3.5 grams. Then we weigh something else and it is
4.2448 grams. If we add them together the answer is 7.7 grams
because we can only have one decimal place. If we multiply them the
answer is 15 grams2 because the answer can only have 2 sig dig.
- Now remember that conversion factors such as (1 L /
1000 mL) DO NOT affect the significant digits because they are exact
numbers.
- More examples:
- 3340 x 1.2 = 4.0 x 103
The answer must have 2 sig dig thus 4000 is incorrect because it only has
1 sig dig.
- 88359 / 3 = 30000
The answer can only have 1 sig dig.
- 8.888 x 3.29853 =
29.32 The answer must have 4 sig dig.
- 1.25 + 3.2 = 4.5
The answer can only have one decimal place.
- 145 - 0.222 = 145
The answer can not have any decimal places.
- 145 - 0.99 = 144
The answer can not have any decimal places.
- 0.042 + 1.33 =
1.37 The answer can have only 2 decimal places.
- More problems
- How many significant
digits are in 23,000?
- How many significant
digits are in 4000.00?
- How many significant
digits are in 0.00023040?
- How many significant
digits are in 400?
- How many significant
digits are in 7.2500?
- How many significant
digits are in 0.00333?
- Write the numbers in
11-16 above in scientific notation.
- Write these numbers in
scientific notation: 42,000; 27.0040;
150,000,000; 0.00000003500; 50,000; 0.0205
- Answers to the above
- 2
- 6
- 5
- 1
- 5
- 3
- 2.3 x 104,
4.00000 x 103, 2.3040 x 10-4, 4 x 102,
7.2500 x 100, 3.33 x 10-3 (Note that you must keep
the correct number of significant digits when putting into scientific
notation
- 4.2 x 104;
2.70040 x 101; 1.5 x 108, 3.500 x 10-8;
5 x 104; 2.05 x 10-2
For conversions within the metric system, you must memorize the conversion (for example: 1000 mL = 1 L, or 1000 g = 1 kg should be memorized) Remember that metric conversions are exact ratios and thus will not limit your significant digits for the answer. First start with what you are given. Figure out what units you need. You will need to create a ratio (conversion factor) between the units given and the units needed. Ask yourself which unit is bigger. Put a "1" by that unit. Then ask how many of the smaller units are in the bigger unit. Put that number in front of the smaller unit. There is your conversion factor. Make sure the units cancel and you get the units you need. Always write your units down. Practice as many of the following as you need - the answers are below.
1 mile = 5280 feet, 12 inches = 1 foot, 3 feet = 1 yard, 1.609 km = 1 mile, 2.54 cm = 1 inch, 1 pound = 0.4536 kg, 1 quart = 946.4 mL.
1. How many pounds does 2.00 kg of cheese weigh?
2. How many mL are in 0.50 quarts?
3. How many inches are in 1.00 km?
4. How many feet are in 3.45 km?
5. How many km are in 5.00 miles?
6. How many yards are in 72.5 miles?
- How many cc's are in 979 mL?
- How many Kelvins are in -15.5oC?
- How many degrees Celsius are in 315 K?
- How many degrees Fahrenheit are in 30.0oC?
- How many Gg are in 4.32 x 1011 g?
- How many meters are in 0.423 Mm?
- How many kL are in 8383 L?
- How many cm are in 0.783 m?
- How many meters are in 252 mm?
- How many grams are in 5232 mg?
- How many liters are in 7.14 x 106 nL?
- How many liters are in 2.52 x 104 mL?
- How many mm are in 0.123 m?
- If the mass of a lead ball is 23.5 g and the volume is
3.5 mL, what is the density of the lead ball?
- If the density of carbon tetrachloride is 0.793 g/mL,
and a sample has a volume of 9.29 mL, what is the mass?
- If the density of ethanol is 0.828 g/mL and a sample
has a mass of 14.5 g what is the volume?
- How many km are in 2.88 m?
- How many cL are in 4.56 x 10-3 L?
- How many ng are in 6.36 x 10-7 g?
- How many Mg are in 4.14 Gg?
- How many mm are in 3.50 x 105 pm?
- How many nL are in 434 µL?
- A water sample of mass 0.0204 kg is how many liters? d
(H2O) = 1.00 g/mL
- If gold's density is 19.32 g/mL, how much would a
0.0333 L sample weigh in grams?
- Table salt has a density of 2.16 g/mL. If you
used 2.00 mL on your food, how much in mg is that?
- The density of ethanol is 0.802 g/mL. How much in
grams does 9.85 x 10-2L mass?
- How many GL are in 2.55 x 107 L?
- How many Mm are in 345 km?
- How many cg are in 0.497 g?
- How many cg are in 2.49 x 103 mg?
- How many mL are in 0.258 L?
- How many µm are in 6.36 x 10-7 m?
- How many nm are in 345 µm?
- What is the density in g/mL of a substance that masses
0.987 kg and has a volume of 4.52 x 102mL?
- How much water in mL would 5.25 mg of copper
displace? d (Cu) = 11.53 g/mL
Answers:
1. 2.00 kg (pound / 0.4536 kg) = 4.41 pounds
2. 0.50 quarts (946.4 mL / quart) = 470 mL
3. 1.00 km (mile / 1.609 km)(5280 feet / mile)(12 inch / ft) = 3.94 x 104 or 39,400 inches
4. 3.45 km (mile / 1.609 km)(5280 ft / mile) = 11,320 or 1.13 x 104 feet
5. 5.00 mile (1.609 km / mile) = 8.05 km
6. 72.5 miles (5280 ft / mile)(yd / 3 ft) = 1.28 x 105 or 128,000 yards
- 979 cc (cc = mL)
- -15.5oC + 273 = 258K
- 315K - 273 = 42oC
- 86oF
- 4.32 x 1011 g (Gg / 109 g)
= 432 Gg
- 0.423 Mm (106 m / Mm) = 4.23 x 105
m
- 8383 L ( kL / 1000 L) = 8.383 kL
- 0.783 m (100 cm / m) = 78.3 cm
- 252 mm (m / 1000 mm) = 0.252 m
- 5232 mg
( g / 106mg)
= 5.232 x 10-3 g
- 7.14 x 106 nL (L / 109 nL) = 7.14
x 10-3 L
- 2.52 x 104 mL (L / 1000 mL) = 25.2 L
- 0.123 m ( 1000mm / m) = 123 mm
- 23.5 g / 3.5 mL = 6.7 g/mL
- 9.29 mL (0.793 g/mL) = 7.37 g
- 14.5 g ( mL / 0.828 g) = 17.5 mL
- 2.88 m ( 1 km / 1000 m) = 2.88 x 10-3
- 4.56 x 10-3 L ( 100 cL / 1 L) = 4.56 x 10-1
or 0.456 cL
- 6.36 x 10-7 g ( 109 ng / 1 g) =
6.37 x 102 or 637 ng
- 4.14 Gg ( 109 g / 1 Gg )( 1 Mg / 106
g) = 4.14 x 103 Mg
- 3.50 x 105 pm ( 1 m / 1012 pm)(
1000 mm / m) = 3.50 x 10-4 mm
- 434 µL ( L / 106 µL)(109 nL / L)
= 4.34 x 105 nL
- Since density of water = 1.000 g/mL: 0.0204 kg (
1000g / kg)(1 mL / 1 g)(L / 1000 mL) = 0.0204 L
- 0.0333 L ( 1000 mL / L )(19.32 g / mL) = 643 g
- 2.00 mL (2.16 g / mL)(1000 mg / g ) = 4320 mg
- 9.85 x 10-2L ( 1000mL / L)(0.802 g / mL) =
79.0 g
- 2.55 x 107 L ( GL / 109 L)
= 2.55 x 10-2 or 0.0255 GL
- 345 km (1000m / km)(Mm / 106 m) = 0.345 Mm
- 0.497 g (100 cg / g) = 49.7 cg
- 2.49 x 103 mg ( g / 1000 mg)(100 cg /
g) = 249 cg or 2.49 x 102 cg
- 0.258 L (1000 mL / L) = 2.58 x 102 or 258 mL
- 6.36 x 10-7 m ( 106µm / m) =
0.636 µm
- 345 µm ( m / 106 µm) ( 109nm
/ m) = 3.45 x 105 nm
- 0.987 kg (1000 g / kg) = 987 g then divide
by 4.52 x 102mL = 2.18 g/mL
- 5.25 mg ( g / 1000 mg)( 1 mL / 11.53 g Cu) = 4.55
x 10-4 mL
Nomenclature Rules
- Binary Ionic Compounds (ionic compounds with two
elements)
- use the metal's name +
the nonmetal's name + the suffix "ide"
- NaCl is sodium
chloride, Li2O is lithium oxide, KBr is potassium bromide, MgI2
is magnesium iodide
- sodium nitride is Na3N,
calcium bromide is CaBr2, aluminum fluoride is AlF3
- note that ionic
compounds are always empirical formulas - we would never say that table
salt is Na6Cl6
- Ionic compounds with polyatomic ions
- treat polyatomic ions as
groups
- never change the name
of the polyatomic ion
- NaOH is sodium
hydroxide, Ca(NO3)2 is calcium nitrate, MgSO4
is magnesium sulfate
- ammonium sulfide is (NH4)2
S, calcium carbonate is CaCO3, magnesium phosphate is Mg3(PO4)2
- Binary Covalent Compounds (covalent compounds with two
elements)
- prefix + nonmetal +
prefix + nonmetal + suffix "ide"
- prefixes are mono, di,
tri, tetra, penta, hexa, hepta, octa
- if there is only one of
the first nonmetal, don't use the mono prefix
- carbon tetraiodide is
CI4, sulfur dioxide is SO2, phosphorus
pentachloride is PCl5
- P2O5
is diphosphorus pentoxide, N2H4 is dinitrogen
tetrahydride, SF2 is sulfur difluoride
- Transition metal compounds: name of transition
metal, (charge in Roman numerals) + name of nonmetal + ide
- Fe2O3
- iron(III) oxide
- CuS - copper(II)
sulfide
- Co(NO3)3
- cobalt(III) nitrate
- FeSO4 -
iron(II) sulfate
- iron(III) flouride -
FeF3
- iron(II) phosphate - Fe3(PO4)2
Oxidation States and Ionic Formulas
Here are some rules about oxidation states, which is a fancy name for charges.
- Alkali metals are always charged +1 when in a
compound. Their goal is to lose one electron, thus becoming +1 cations.
- Alkaline Earth metals are always charged +2 when
in a compound. Their goal is to lose two electrons, thus becoming +2
cations.
- B column is usually charged +3 or -5 depending on what
they are bonded to. They can lose 3 electrons becoming +3 OR gain 5
electrons becoming -5. Actually Al, Ga and In are always
+3.
- C column is usually charged +4 or -4 depending on what
it is bonded to. They can lose 4 electrons becoming +4 OR gain 4
electrons becoming -4. (They can also be +2 or -2.) Example CH4
C is -4 and H is +1. Example CO2 C is +4 and O is
-2.
- N column is usually charged -3 in compounds but
sometimes +5. They can lose 5 electrons becoming +5 OR gain 3
electrons becoming -3. Example NH3 N is -3 and H is
+1.
- Chalcogens are usually charged -2 in compounds (Oxygen
is always charged -2 when in a compound except for peroxides like H2O2
where it is -1) unless they are bonded to something more electronegative
than they are, in this case they can be +6 +4 or +2. Example sulfate
ion. SO42- Here the total charge is -2 so
S is +6, each O is -2 (there are 4 of them so that adds up to -8 for all
four O's) so that adds up to -2 overall, which is why sulfate is an ion
and not a molecule - it has an overall charge.
- Halogens are usually charged -1 in compounds (F always
is -1) unless they are bonded to something more electronegative than they
are, in this case they can be +7 +5 or +3. Example IF7.
Here F is -1 since it always is -1. That makes I +7. Note that
IF5 and IF3 also exist.
- Noble gases are stable and unreactive, they don't often
have a charge - they are neutral atoms.
- Transition metals have many charges, too many to
predict. Exceptions are Zn and Cd which are always +2 and
silver which is always +1. My research focused on Re which
can be charged from +1 all the way up to +9.
- H is usually +1. When H is bonded to a metal
however, it can be -1. Example NaH where Na is +1 since it is always
+1 so H must be -1.
- Remember the atoms in a pure element have a charge of
ZERO!!! Atoms don't have charges unless they are bonded to something
else.
OK, armed with this information, Let's predict chemical formulas for compounds from the following and name them:
- lithium and oxygen
- magnesium and phosphate
- ammonium and sulfur
- calcium and fluorine
- aluminum and chlorine
- potassium and nitrate
- sodium and sulfate
- phosphorus and oxygen
- silicon and hydrogen
- calcium and hydroxide
- silver and carbonate
- sodium and bromine
Answers
- Li2O (Li is +1 so we need 2 of them
since O is -2) lithium oxide
- Mg3(PO4)2 (Mg is
+2 so we need 3 of them, phosphate is -3 so we need 2 of them) magnesium
phosphate
- (NH4)2S (ammonium is
+1 so we need 2 of them since S is -2) ammonium sulfide
- CaF2 (Ca is +2 and F is -1 so we need
two F) calcium fluoride
- AlCl3 (Al is +3 and Cl is -1 so we
need 3 of them) aluminum chloride
- KNO3 ( K is +1 and nitrate is
-1) potassium nitrate
- Na2SO4 (Na is +1 so we need 2 of
them since sulfate is -2) sodium sulfate
- P2O5 (O is -2 according to
the rules, so P must be +5 and not -3 since negatives repel, we need 2 P's
to make +10 and 5 O's to make -10) diphosphorus pentoxide
- SiH4 (Since H is +1, Si must be -4,
and so we need 4 H's) silicon tetrahydride
- Ca(OH)2 (Ca is +2 and OH is -1 so we
need 2 of them) calcium hydroxide
- Ag2CO3 (Ag is +1 so we need
2 of them since carbonate is -2) silver carbonate or I'd also accept
silver(I) carbonate
- NaBr (Na is +1 and Br is -1) sodium bromide
You should know these polyatomic ions by the way!
- sulfate SO42-
- phosphate PO43-
- carbonate CO32-
- hydroxide OH-
- ammonium NH4+
- acetate CH3COO-
- nitrate NO3-
- bicarbonate HCO3-
- monohydrogen phosphate HPO42-
- dihydrogen phosphate H2PO4-1
The following practice problems deal with converting between moles and atoms or molecules using Avagadro's Number. Then we'll also convert between grams and atoms or molecules using Avogadro's Number and Molar Mass. Remember if we start with an entire molecule but want to end with just atoms (or vice versa), we need to use molar ratios such as ( 2 H atoms / 1 H2O molecule).
- How many atoms are in 4.00 moles of carbon?
- How many moles are in 1.25 x 1025 atoms of
neon?
- How many molecules are in 2.99 moles of ammonia?
- How many moles are in 5.25 x 1024 molecules of
lithium sulfide?
- How many molecules are in 4.24 moles of sodium
chloride?
- How many H atoms are in 8.35 moles of water?
- How many O atoms are in 1.44 moles of magnesium
sulfate?
- If I have 3.45 x 1025 H atoms in a pure CH4
sample, how many moles of CH4 are present?
- How many molecules are in 5.25 grams of LiBr?
- How much does 6.46 x 1025 molecules of CaS
mass?
- How many atoms are in 24.7 grams of argon?
- How much does 3.33 x 1024 nitrogen atoms
mass?
- How many molecules are in 34.5 grams of calcium
phosphate?
- How many C atoms are in 2.64 grams of C2H4?
- How many Br atoms are in 14.3 grams of bromine?
- A sample of silicon dioxide contains 5.25 x 1026
oxygen atoms. How much does this sample mass?
Answers
- 4.00 mol C (6.02 x 1023 atoms / mol) = 2.41
x 1024 C atoms
- 1.25 x 1025 atoms Ne ( mol / 6.02 x 1023
atoms) = 20.8 mol Ne
- 2.99 mol NH3 (6.02 x 1023
molecules / mol) = 1.80 x 1024 molecules NH3
- 5.25 x 1024 molecules Li2S (mol /
6.02 x 1023 molecules) = 8.72 mol Li2S
- 4.24 mol NaCl (6.02 x 1023 molecules / mol)
= 2.55 x 1024 molecules NaCl
- 8.35 mol H2O ( 2 H / 1 H2O) (6.02
x 1023 atoms / mol) = 1.01 x 1025 H atoms
- 1.44 mol MgSO4 ( 4 O / MgSO4)
(6.02 x 1023 atoms / mol) = 3.47 x 1024 O atoms
- 3.45 x 1025 H atoms ( 1 CH4
molecule/ 4 H atoms) (mol / 6.02 x 1023 molecules) = 14.3 mol
CH4
- 5.25 g LiBr (mol / 86.8 g)(6.02 x 1023
molecules / mol) = 3.64 x 1022 molecules LiBr
- 6.46 x 1025 molecules CaS (mol / 6.02 x 1023
molecules)(72.2 g / mol) = 7750 g CaS
- 24.7 g Ar ( mol / 39.9 g) (6.02 x 1023 atoms
/ mol) = 3.73 x 1023 atoms Ar
- 3.33 x 1024 N atoms (mol / 6.02 x 1023
atoms) (14.0 g / mol ) = 77.4 g N
- 34.5 g Ca3(PO4)2 (mol
/ 310.3 g) (6.02 x 1023 molecules / mol) = 6.69 x 1022
molecules Ca3(PO4)2
- 2.64 g C2H4 ( mol / 28.0 g) (2 C
/ 1 C2H4) (6.02 x 1023 atoms / mol) =
1.14 x 1023 C atoms
- 14.3 g Br2 ( mol / 159.8 g) (2 Br / Br2)
(6.02 x 1023 atoms / mol) = 1.08 x 1023 Br atoms
- 5.25 x 1026 O atoms (mol / 6.02 x 1023
atoms) (1 SiO2 / 2 O ) ( 60.1 g / mol) = 2.62 x 104
g SiO2
Remember when writing reactions, you must have all the correct formulas for the reactant(s) and product(s) first. Formulas depend on the charges of the elements and polyatomic ions involved. Once you have determined the formulas, you can NOT change them later - don't change the subscripts in the formulas. For example sodium and chlorine make NaCl. You cannot later while balancing change this to NaCl2. You can only add coefficients in front of the compounds to balance the reaction - like 2 NaCl.
Formation rxn: reactants are elements in their natural state forming a product compound
Combustion rxn: hydrocarbon (molecule with only C and H) adding to oxygen producing water and carbon dioxide
Precipitation
rxn: (a type of double replacement) when the cations swap anions and one
product is NOT soluble
Acide Base rxn: Where the H+ from an acid comes off and the base (often hydroxide ion) gains the H+
- Balance the following formation reaction: Ca (s)
+ I2 (s) --> CaI2
- Balance the following formation reaction: Na (s)
+ Br2 (l) --> NaBr
- Write and balance the formation reaction for magnesium
and chlorine.
- Write and balance the formation reaction for potassium
and oxygen.
- Write and balance the formation reaction for calcium
and oxygen.
- Write and balance the formation reaction for phosphorus
and chlorine.
- Balance the following combustion reaction: C4H8
+ O2 (g) --> CO2 (g) + H2O (g)
- Balance the following combustion reaction: C6H14
+ O2 (g) --> CO2 (g) + H2O (g)
- Write and balance the combustion reaction for C6H6.
- Write and balance the combustion reaction for C3H6.
- Balance the following double displacement
reaction: CaI2 + Na2SO4 --> CaSO4
+ NaI
- Balance the following double displacement
reaction: MgSO4 + KNO3 --> Mg(NO3)2
+ K2SO4
- Write and balance the double displacement reaction for magnesium
chloride and potassium oxide.
- Write and balance the double displacement reaction for
calcium nitride and lithium bromide.
- Write and balance the double displacement reaction for
silver nitrate and iron(III) chloride.
Answers
- already balanced
- 2 Na (s) + Br2 (l) --> 2 NaBr
- Mg (s) + Cl2 (g) --> MgCl2
- 4 K (s) + O2 (g) --> 2 K2O
- 2 Ca (s) + O2 (g) --> 2 CaO
- 2 P (s) + 5 Cl2 (g) --> 2 PCl5
- C4H8 + 6 O2 (g) -->
4 CO2 (g) + 4 H2O (g)
- 2 C6H14 + 19 O2 (g)
--> 12 CO2 (g) + 14 H2O (g)
- 2 C6H6 + 15 O2 (g)
--> 12 CO2 (g) + 6 H2O (g)
- 2 C3H6 + 9 O2 (g)
--> 6 CO2 (g) + 6 H2O (g)
- CaI2 + Na2SO4 -->
CaSO4 + 2 NaI
- MgSO4 + 2 KNO3 --> Mg(NO3)2
+ K2SO4
- MgCl2 + K2O --> MgO + 2 KCl
- Ca3N2 + 6 LiBr --> 3 CaBr2
+ 2 Li3N
- 3 AgNO3 + FeCl3 --> 3 AgCl +
Fe(NO3)3
Molarity and dilution
Molarity (M) = moles per liter (mol/L) Note you may need to change grams to moles using the molar mass first.
Dilution equation: M1V1 = M2V2 where 1 is before and 2 is after, thus V2 is the total final volume
- How many moles are in 234 mL of a 1.29 M solution?
- What is the molarity if 9.25 moles of sodium sulfide is
dissolved to make a 787 mL solution?
- What is the molarity if 43.2 grams of calcium chloride
is dissolved to make a 1145 mL solution?
- How many grams do you need to prepare 0.500 L of a 4.00
M solution of sodium bromide?
- The salt is blood serum is 0.14 M. How many grams
of sodium chloride are in 5.00 mL of blood serum?
- I want to make a 0.555 M potassium iodide
solution. I have 12.7 grams of potassium iodide and I want to use it
all. What will be the volume of my solution?
- I used 54.7 grams of sodium phosphate to make a 0.250 M
solution. What is the volume in mL?
- I have 20.0 mL of a 2.34 M solution. If I add
15.0 mL to the solution, what is the final molarity?
- I had 25.0 mL of a 5.00 M solution. How much
water did I add if I diluted the solution to 2.30 M?
- What is the final volume of a 0.833 M solution if the
original was 10.0 mL of a 1.00 M solution?
- What was the original molarity of 25.0 mL of a 0.424 M
solution if the original volume was 10.0 mL?
- Concentrated HCl is 6.00 M. In lab we used 1.00 M
HCl. If we need 500.0 mL in lab, how much of the concentrated do I
need to dilute?
Answers
- 1.29 mol / L = x moles / 0.234 L, x = (1.29
mol/L)(0.234 L) = 0.302 moles
- M = (9.25 moles / 0.787 L) = 11.8 M
- 43.2 g CaCl2 (mol / 111.1 g) = 0.389 moles,
M = (0.389 mol / 1.145 L) = 0.340 M
- (0.500 L )(4.00 mol/L) = 2.00 moles NaBr needed, 2.00
mol NaBr (102.9 g/mol) = 206 grams
- (0.14 mol/L)(0.00500 L) = 7.00 x 10-4 moles,
7.00 x 10-4 moles NaCl (58.5 g/mol) = 0.0410 g
- 12.7 g KI (mol / 166.0 g) = 0.0765 moles
KI, 0.0765 mol ( 1 L / 0.555 mol) = 0.138 L
- 54.7 g Na3PO4 (mol / 164 g) =
0.334 moles, 0.334 mol ( 1 L / 0.250 mol) = 1.34 L = 1340 mL
- (2.34 M)(20.0 mL) = M2 (35.0 mL), M2
= 1.34 M
- (5.00 M)(25.0 mL) = (2.30 M) V2, V2
= 54.3 mL so I added 29.3 mL
- (10.0 mL)(1.00 M) = (0.833 M) V2,
V2 = 12.0 mL
- M1 (10.0 mL) = (0.424 M)(25.0 mL)
, M1 = 1.06 M
- (6.00 M) V1 = (1.00 M)(500.0
mL), V1 = 83.3 mL
Use the following balanced chemical reaction to answer practice questions 1 - 11.
H2SO4 + 2 NaOH g Na2SO4 + 2 H2O
1. How many moles of water can be produced from 0.234 moles H2SO4 ?
2. How many moles of sodium hydroxide are needed to completely react with 0.878 moles H2SO4 ?
3. How many moles of sodium hydroxide were used to produced 1.32 moles of sodium sulfate?
4. How many moles of H2SO4 are needed to completely react with 3.13 grams of sodium hydroxide?
5. How many grams of sodium sulfate could be produced from 0.525 mol sodium hydroxide?
6. How many moles of water could be produced from 25.0 grams H2SO4 ?
7. How many grams of sodium sulfate could be produced from 13.8 grams H2SO4 ?
8. How many grams of sodium hydroxide were needed to produce 65.0 grams of water?
9. How many grams of sodium hydroxide are needed to completely react with 21.3 grams H2SO4 ?
10. How many grams of water were produced if 47.0 grams of sodium sulfate were also produced?
11. How many grams of sodium sulfate can be produced from 23.7 grams H2SO4 and 23.7 grams sodium hydroxide?
Answers
1. 0.234 mol H2SO4 (2 H2O / 1 H2SO4 ) = 0.468 mol H2O
2. 0.878 mol H2SO4 (2 NaOH / 1 H2SO4 ) = 1.76 mol NaOH
3. 1.32 mol Na2SO4 (2 NaOH / 1 Na2SO4 ) = 2.64 mol NaOH
4. 3.13 g NaOH (mol / 40.0 g)(1 H2SO4 / 2 NaOH) = 0.0391 mol H2SO4
5. 0.525 mol NaOH (1 Na2SO4 / 2 NaOH)(142.1 g / mol) = 37.3 g Na2SO4
6. 25.0 g H2SO4 (mol / 98.1 g)(2 H2O / 1 H2SO4 ) = 0.510 mol H2O
7. 13.8 g H2SO4 (mol / 98.1 g)(1 Na2SO4 / 1 H2SO4 )(142.1 g / mol) = 20.0 g Na2SO4
8. 65.0 g H2O (mol / 18.0 g)(2 NaOH / 2 H2O)(40.0 g / mol) = 144 g NaOH
9. 21.3 g H2SO4 (mol / 98.1 g)(2 NaOH / 1 H2SO4 )(40.0 g / mol) = 17.4 g NaOH
10. 47.0 g Na2SO4 (mol / 142.1 g)(2 H2O / 1 Na2SO4 )(18.0 g / mol) = 11.9 g H2O
11. This is a limiting reagent problem, so we work both out: 23.7 g H2SO4 (mol / 98.1 g)(1 Na2SO4 / 1 H2SO4 )(142.1 g / mol) = 34.3 g Na2SO4 possible OR 23.7 g NaOH (mol / 40.0 g)(1 Na2SO4 / 2 NaOH )(142.1 g / mol) = 42.1 g Na2SO4 possible . Since 34.3 grams is the smaller of the two possible amounts, it is the real answer which means H2SO4 is the limiting reagent and NaOH is in excess.
Now answer the following stoichiometric problems.
1. Write and balance the formation reaction between nitrogen and hydrogen. If I start with 9.33 grams of nitrogen, how much product can I make? If I actually made 9.74 grams of product, what was my percent yield?
2. Write and balance the formation reaction that produces phosphorus pentachloride. How many grams of chlorine do I need in order to produce 1.42 grams of phosphorus pentachloride?
3. Write and balance the combustion reaction for the hydrocarbon C6H12. How many grams of oxygen will I need to completely combust 534 grams of C6H12?
4. Write and balance the metathesis reaction between calcium phosphate and potassium bromide. If I produced 42.4 grams of calcium bromide, how many grams of calcium phosphate did I start with?
5. Write and balance the metathesis reaction between sodium chloride and potassium fluoride. If I start with 66.2 grams potassium fluoride, how many grams of sodium fluoride can I produce? If I actually produce 35.7 grams of sodium fluoride, what is my percent yield?
6. Write and balance the formation reaction for carbon dioxide. If I start with 1.77 grams carbon, how much product can I make? If I actually produce 2.10 grams of product, what is my percent yield?
7. If my theoretical yield is 10.0 grams and my actual yield is 7.24 grams, what is my percent yield?
8. If my theoretical yield is 10.0 grams and my actual yield is 11.24 grams, what is my percent yield?
9. How many grams of potassium sulfate can be produced by the metathesis reaction of 8.88 grams potassium chloride and 4.44 grams magnesium sulfate?
Answers
1. N2 (g) + 3 H2 (g) g 2 NH3 (g) 9.33 g N2 (mol / 28 g)(2 NH3 / 1 N2)(17.0 g / mol) = 11.3 g NH3 is what I could make. However, my percent yield is (9.74 / 11.3) x 100 = 86.2%
2. 2 P (s) + 5 Cl2 (g) g 2 PCl5 1.42 g PCl5 (mol / 208.5 g)( 5 Cl2 / 2 PCl5)(71.0 g / mol) = 1.21 g Cl2
3. C6H12 + 9 O2 (g) g 6 CO2 (g) + 6 H2O (g) 534 g C6H12 (mol / 84.0 g)(9 O2 / 1 C6H12 )(32.0 g / mol) = 1830 g O2
4. Ca3(PO4)2 + 6 KBr g 2 K3PO4 + 3 CaBr2 42.4 g CaBr2(mol / 199.9 g)(1 Ca3(PO4)2 / 3 CaBr2 )(310.3 g / mol) = 21.9 g Ca3(PO4)2
5. NaCl + KF g NaF + KCl 66.2 g KF (mol / 58.1 g)(1 NaF / 1 KF)(42.0 g / mol) = 47.9 g NaF my percent yield is (35.7 / 47.9) x 100 = 74.5%
6. C (s) + O2 (g) g CO2 (g) 1.77 g C (mol / 12.0 g)(1 CO2 / 1 C)(44.0 g / mol) = 6.49 g CO2 my percent yield is (2.10 / 6.49) x 100 = 32.4%
7. (7.24 / 10.0) x 100 = 72.4%
8. (11.24 / 10.0) x 100 = 112.4% - Something is wrong here. I can't create matter. When percent yield is higher than 100% there is a human error. Perhaps I weighed more reactant out than I realized. Perhaps water condensed inside my reaction vessel. Perhaps my theoretical yield calculation was wrong.
9. First write the reaction: 2 KCl + MgSO4 g K2SO4 + MgCl2 Now this is a limiting reagent problem. Calculate the answer based on 8.88 g KCl: 8.88 g KCl ( mol / 74.6 g)(K2SO4 / 2KCl)(174.3 g/mol) = 10.4 g K2SO4 . Now calculate the answer based on 4.44 g MgSO4 : 4.44 g MgSO4 ( mol / 120.4g)( K2SO4 / MgSO4 )(174.3 g/mol) = 6.43 g K2SO4 . Compare and the answer is really 6.43 g, magnesium sulfate is the limiting reagent and potassium chloride is in excess.
reduction = gaining electrons, since electrons are negative the charge goes down, when a chemical is reduced it is called the oxidizing agent
Oxidation = losing electrons, when electrons go away the atom is more positive so the charge goes up, when a chemical is oxidized it is called the reducing agent
exothermic = heat is produced, heat is a product, the reaction gets warm as heat is released
endothermic = heat is need to react, heat is a reactant, the reaction feels cold as it absorbs surrounding heat
- Is the reaction occurring in an instant hot pack
exothermic or endothermic?
- What is the charge on sulfur in H2SO4?
- What is the charge on iron in FePO4?
- What is the charge on Cl in Cl2 (g)?
- What is the charge on a sodium atom in Na (s)?
- For the following reactions, identify what is oxidized,
what is reduced, what is the oxidizing agent, and what is the reducing
agent:
- 3 Ni (s) + 2 Al3+ (aq)
--> 3 Ni2+ (aq) + 2 Al (s) (Note the reaction
is balanced so that the charge on each side of the arrow is equal)
- Cu2+ (aq) +
Cd (s) --> Cu (s) + Cd2+ (aq)
- 2 Na (s) + 2 H2O
(l) --> 2 NaOH (aq) + H2 (g)
- 2 Li (s) + I2
(s) --> 2 LiI (s)
- 4 Al (s) + 3 O2
(g) --> 2 Al2O3 (s)
- 2 CuNO3 +
PdCl4 --> 2 CuCl2 + Pd(NO3)2
Answers
- exothermic - it is producing heat
- +6 (O is -2 and there are four of them, H is +1 so that
adds up to -6 thus S must be +6)
- +3 (phosphate ion is -3 so iron must be +3)
- zero (all elements are zero)
- zero (it's an element)
- Answers:
- oxidized = reducing
agent = Ni (s), reduced = oxidizing agent = Al3+ (aq)
- oxidized = reducing
agent = Cd (s), reduced = oxidizing agent = Cu2+ (aq)
- oxidized = reducing
agent = Na (s), reduced = oxidizing agent = H2O (l)
- oxidized = reducing
agent = Li (s), reduced = oxidizing agent = I2 (s)
- oxidized = reducing
agent = Al (s), reduced = oxidizing agent = O2 (g)
- oxidized = reducing
agent = CuNO3 , reduced = oxidizing agent = PdCl4
Quantum Numbers, shells, orbitals
- Shells, Subshells and Orbitals
- Shells are three
dimensional spheres around the nucleus. They are numbered 1, 2, 3,
4, 5, 6, 7... Make sure you understand they are spheres not circles
- imagine the nucleus as a tiny steel ball bearing. The shells
would be like a ping pong ball around the steel ball, then a baseball,
then a soccer ball, then a beach ball, etc... The shells are quantized
because nothing exists between them. Electrons must be on a shell,
not in between. Stairs are also quantized, ramps are not.
- Subshells are on the
shells. We have s, p, d, f, g... subshells. Shell one has one
subshell - s. Shell 2 has 2 subshells - s and p. Shell 3 has
3 subshells - s, p, d. Shell 4 has 4 subshells - s, p, d, f.
And so forth.
- Subshells consist of
orbitals. The s subshell has one orbital. The p subshell has
3 orbitals. The d subshell has 5 orbitals. The f subshell has
7 orbitals. And so forth with odd numbers of orbitals.
- Two electrons can fit
into one orbital, but they must have opposite spins. Electrons are
always spinning.
- Putting it together:
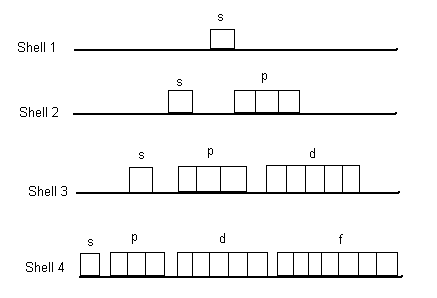
- Shell one - one
subshell (s), one orbital, 2 electrons maximum.
- Shell two - two
subshells (s, p), 4 orbitals, 8 electrons maximum.
- Shell three - three
subshells (s, p, d), 9 orbitals, 18 electrons maximum.
- Shell four - four
subshells (s, p, d, f), 16 orbitals, 32 electrons maximum.
- Example questions
- How many subshells are
on the fourth shell? 4
- How many orbitals are
on the third shell? 9
- How many orbitals are
in a p subshell? 3
- How many electrons can
fit into a d subshell? 10
- How many orbitals are
in an f subshell? 7
- How many electrons can
fit into a p orbital? 2
7. Shells exist around the nucleus at certain distances. There is nothing between the shells and electrons can not exist anywhere but on the shells. We use this word to describe the shells and how they exist. answer: quantized
8. How many subshells are on the third shell? 3
9. How many electrons can fit into an orbital? 2
10. How many orbitals are on the first shell? 1
11. How many electrons maximum can fit on the fourth shell? 32
- Quantum Numbers
- A quantum number is an address
for an electron. No two electrons have the same address. A quantum
number looks like this: (n, l, ml, ms)
where n = shell number, l = subshell number, ml
= orbital number and ms = spin number.
- Shell number n.
Simply 1, 2, 3, 4, 5, 6, 7, ...
- Subshell number l. We have to
assign numbers to the subshells. We let 0 = s, 1 = p, 2 = d, 3 = f,
etc. Note that l must be less than n.
For example, if n = 3, then l must be 2, 1, or 0
because the third shell only has s, p, and d subshells, not an f subshell
- so l
can not be 3 or higher.
- Orbital number ml.
We let the middle orbital be number 0 and then number the other orbitals
if there are any.
- When l = 0, it is the s
subshell, there is only one orbital, it is numbered 0.
- When l = 1, it is the p subshell,
there are three orbitals, they are numbered -1, 0, 1.
- When l = 2, it is the d
subshell, there are five orbitals, they are numbered -2, -1, 0, 1, 2.
- When l = 3, it is the f
subshell, there are seven orbitals, they are numbered -3, -2, -1, 0, 1,
2, 3.
- So if an electron is
in a p subshell, what are the possible ml numbers? -1,
0, 1
- So if an electron is
in a f subshell, what are the possible ml numbers? -3,
-2, -1, 0, 1, 2, 3.
- Note that ml
possible values are from -l to +l. For example,
if l
= 2 the possible ml values are -2, -1, 0, 1, 2.
- Spin number ms.
We assign +1/2 to a clockwise spin and -1/2 to a counter clockwise
spin. So the possible ms numbers are just -1/2 and
+1/2. Nothing else.
- Entering a number in scientific notation: Let's enter 3.53
x 108 in our calculator. Push in [3.53] then push your
[EE] or [EXP] button which stands for "times ten to the power
of" all in that one little button. Then push in [8].
There you have it. Now try 2.52 x 10-23. Push in
[2.52] then [EE] or [exp] then [+/-] to get the negative sign, then
[23]. There you have it.
- Making your calculator display numbers in scientific
notation: Punch in the number 234000. Now there are 2 main
ways to make your calculator display this in scientific notation depending
on your calculator. (1) Push the [2nd] function button. Now
find the button that says SCI over the top of it. It is usually the
[8 or 5] key. Now your calculator should show 2.34 EE 5 or something
similar. Graphing calculators do it like this (2) Find the mode
button on your calculator and push it. Move the cursor by pushing
the arrow buttons until the cursor highlights the SCI mode. Push
[enter]. Now quit the mode selection process by pushing [quit].
Now your calculator should show the number in scientific notation like
2.34 EE 5 or something
similar.
- NEVER EVER ENTER SCIENTIFIC NUMBERS BY ACTUALLY PUSHING
IN [x] [10] [^] or [yX] BUTTONS. Always use the [EE] or
[EXP] buttons.
- Calculations with scientific notation: Try the following
calculations.
- (2.55 x 108
)(8.21 x 104) = 2.09 x 1013
- (4.24 x 10-4)(7.51
x 10-5) = 3.18 x 10-8
- (4.77 x 103)
/ (2.24 x 10-7) = 2.13 x 1010
- 525 / [(2.24 x 103)(8.35
x 10-3)] = 28.1
- 17.3 (7.13 x 10-23)
/ [(77.34)(6.26 x 10-18)] = 2.55 x 10-6
Did any of the answers have less digits than you had? That's due to SIGNIFICANT DIGITS. When multiplying and dividing the answer can only have the same amount of digits as the number in the problem with the least amount of significant digits. Your answer can never be more significant than your given information. Thus 6a and 6c has 2 digits while 6b and 6d has 3 digits in the answer. Now remember that conversion factors such as (1 L / 1000 mL) DO NOT affect the significant digits because they are exact numbers. Note than when adding and subtracting you focus on decimal places. Your answer can not have more decimal places than the number with the least amount of decimal places. Try some more.
- ([24.5)(0.084852)] / [(12.2)(2.234)] = 0.0763 is the
answer. If you got 0.381 you put 2.234 in the numerator instead of
the denominator. Here is what you actually push into the
calculator: (24.5) times (0.084852) divided by (12.2) divided by
(2.234). If you push times before 2.234 the calculator puts it in
the numerator .
- (1.24 x 10-19)(3.15 x 1013) =
3.91 x 10-6 is the answer. If you got the 3.91 correct
but the wrong power of ten you used scientific notation incorrectly in
your calculator. Actually push in this: 1.24 EE (+/-)19 times
3.15 EE 13 = and you should get the correct answer. To make your
calculator show you the answer in scientific notation, for most
calculators push 2nd function then SCI.
- [(3.2 x 10-29)(3.776 x 1014)] /
[(8.3 x 109)(5.25 x 1021)] = 2.8 x 10-46
(Only 2 significant digits because of all the numbers the least amount of
sig dig was 2)
- (231.5) / [(1.2345 x 103)(9.8765 x 10-4)]
= 189.9
- 3340 x 1.2 = 4.0 x 103 The answer must
have 2 sig dig thus 4000 is incorrect because it only has 1 sig dig.
- 88359 / 3 = 30000 The answer can only have 1 sig
dig.
- 8.888 x 3.29853 = 29.32 The answer must have 4
sig dig.
- 1.25 + 3.2 = 4.5 The answer can only have one
decimal place.
- 145 - 0.222 = 145 The answer can not have any
decimal places.
- 145 - 0.99 = 144 The answer can not have any
decimal places.
- 0.042 + 1.33 = 1.37 The answer can have only 2
decimal places.
For 152 students.
- Try the following problems using logs. However
many significant digits are in the number for which you take the log are
how many decimal places your answer should have. So log 4.11 should
have an answer with 3 decimal places = .614. Log 46 should have an
answer with only 2 decimal places = 1.66. Remember log is base 10,
ln is natural log. You should know the difference.
- log 5.2 = 0.72
- log (4.27 x 10-4)
= -3.370
- 9.2 = log
x, solve for x, x = 2 x 109
(you have to use the [2nd] function and [log] buttons to raise both sides
to the 10.)
- 4.25 = -log x, solve
for x, x = 5.6 x 10-5
- 13.45 = -log x, solve
for x, x = 3.5 x 10-14
- ln 3.55 x 1084
= 1.947 x 102
- 9.25 x 10-3
= ln x, solve for x, x = 1.009
- How do you solve 259.403 = ln x. Solving for x
> 1.0 x 1099. Sometimes x is so huge our calculators
can't display it. Here's what you do:
- Example: ln x = 200,
Solve by taking e both sides, x = 7.23 x 1086
- Eventually the exponent
will be greater than 99 and our calculators don't show more than 2 digits
in a calculator - so must solve like this: ln x = 300, If you take
e of both sides you get ERROR
So ln x = 150 + 150 (divide the large number of 300 by 2 so you have smaller numbers)
take each number to e
x = (1.39 x 1065)(1.39 x 1065) and solve by doing 1.39 squared then adding the exponents
x = 1.93 x 10130 COOL!!! - So the answer for the
above question where ln x = 259.403 is x = 4.54 x 10112
![]()
![]()
![]()
There are 4 main reasons student don't like or do poorly in chemistry:
1. They get behind and never catch up
2. They don't know how to use their scientific calculator
3. They don't spend enough time on the material
4. They don't practice problems
How to Pass Chemistry:
1.
Notetaking: Take notes during
lecture. If you miss a class, the
lecture closely follows the review, also try to get notes from a classmate.
2.
You should read over your lecture notes later that SAME day. Before you go to bed or after dinner, read
over the notes you took that very day.
This increases your chance of remembering them by 50%!!!
3.
You should take your own notes as you read each chapter. You will then have not only lecture notes and
the review, but your own personal notes for each chapter. Group them together in your notebook.
4.
Do NOT wait until exam time to look at your notes for the first time since
taking them. By that time you will not
even remember writing those notes that first week of school! If you wait until test time, you will look
back at your notes and not even remember writing them! When the professor finishes a chapter, you
should read ALL the notes on that chapter again, and maybe even again!
5.
Try to read ahead of the professor.
Lectures are often fast paced and may sometimes be boring. It really helps if you have already seen the
material, even quickly, before going to lecture. This makes it much easier to
follow along and stay interested. Read
the material before lecture trying to understand it as well as you can on your
own. Then the professor may make it all
come together. Otherwise the professor
may go so quick that you are completely lost.
6.
NEVER GET BEHIND! Catching up in chemistry is near
impossible.
7.
Study in 30 minute intervals several days every week. Studies prove you remember the first and last
15 minutes you study well, so study 30 minutes!
Take many breaks like call someone, write an email, listen to a song, or
change to a non-scientific subject like English. Telling yourself that you will sit down on
the weekend and study chemistry for 5 hours straight is a lie! Study a little bit almost every day, do not
cram. Do not study chemistry only on
Sunday for example. You will get burned
out on Sunday and forget too much in between.
8.
Work end of the chapter problems that are answered in the back of the text so
you can check yourself. Try online
tutorials and interactive problems.
Don't work problems that you have no answer for because you may do it
wrong and thus learn incorrectly.
9. Ask for help always!!! Bug your professor. Don't let questions go unanswered!
Test Taking Strategies
1. Stay on top of
the course so you don't have to cram.
Don't cram for an exam.
2. Do not pull all
nighter's on coffee or vivarin. You'll
be dead walking by the exam and will not perform well. Also you quickly forget what you cram, so
when the next test builds on previous knowledge (and the final is cumulative)
you will be starting from square one again.
3. You can only really
focus on one subject for about 1.5 hours.
So if you study the hour before the exam, your brain will shut down on
you before the exam is finished. One
hour before the exam, close your book and notes. You're done studying! If you don't understand it now, it is too
late anyway. Chill out for this hour,
let your brain rest so it is recharged for the exam. Watch TV, eat dinner, chat with friends. Do anything but study!
4. Study with a
group. Divide up the material and have
each person present their part to the group.
5. Study alone as
well. Read all your notes. Read the reviews. Do practice
problems. Look over quizzes and homework. I primarily write the exams
from the chapter reviews posted on this web page.
6. Study in 30 minute
intervals with breaks. Do not attempt to
study for 3-4 hours straight because you will burn out and get bored.
7. Try to study for an
exam several nights, not just the night before.
If you have a question, it will be too late to get help the night before
most likely. Plan ahead and study several days before each exam.
Why should I practice? I followed my professor in lecture just fine!
Doing a problem on your own is NOT the same as following your professor along in class. It is easy to understand a problem when you simply watch and listen along while your professor does all the work. Trying a similar problem may not be as easy as you think - which is why you must practice!
I hear this all the time: "You made it seem so easy in class. I totally understood it when you did this in class." Yet the student can not answer the problem on his/her own.
We professors often don't have time in class to let you practice many problems - we typically work one example or two, then maybe give you a chance to work one problem on your own. That is not enough - please work many practice problems on your own outside of class. You will learn and perform much better.
Good Luck!